Increased Number of Megakaryocytes in the Synovium and Cartilage of Arthritic Mouse Joints
Abstract
Bone remodeling processes in rheumatoid arthritis (RA) depend mainly on the action of three types of cells. Osteoblasts are responsible for the formation of new bone, osteoclasts degrade mineralized bone and osteocytes regulate and maintain the bone homeostasis. Except, many other cell populations become pathologically activated in the inflamed microenvironment of the joint. The role of megakaryocytes and platelets in RA is poorly clarified. In the present study the presence of MK in the synovium and cartilage was observed in a model of arthritis induced in normal and complement depleted mice.
Author Contributions
Academic Editor: Chabchoub Ghazi, National Health Insurance of Tunisia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Petya Ganova, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Little is known about the interactions of bone cells and megakaryocytes (MK) within the bone marrow. The MKs are highly specialized precursors for platelets which under certain conditions, modulate bone formation and resorption by the production of regulatory proteins. Megakaryocytes present in very low numbers comprising 0.03–0.06% of the total nucleated cells in human bone marrow. For this reason, they have proved not to be the population investigated systematically. Their size varies between 20 and 30 μm with abundance of cytoplasm and the full maturation of MK continues 72 h when 1000–5000 platelets being produced from each megakaryocyte1. Available data show that MK may play dual and opposing roles in osteoclastogenesis according to the expression of Receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegerin (OPG). When MKs express RANKL it could enhance osteoclast (OC) development, while other investigations reported the expression of OPG in MKs which suppose inhibition of OC development2, 3. Although there is increasing evidence for interactions between MKs and osteoblasts, the effects of these interactions in humans have not been reported. In vitro studies demonstrated that isolated and enriched megakaryocytes attach to bone marrow fibroblasts and generate an increased growth of these cells4. It is of interest whether a platelet can support or inhibit inflammation since it contains both pro-inflmmtory and anti-inflammatory cytokines such as P-selectin, CD40L, IL-1β, transforming growth factor-β (TGF-b) and thrombospondin-1, and express several functional Toll-like receptors5, 6. Upon platelet activation P-selectin (CD62P) is translocated from the a-granule to the platelet surface. It has been reported that P-selectin plays an important role in the development of the Th-1 immune responses7. Data about the role of complement system on MK maturation and function are too scarce. Excessive activation of complement system significantly contributes to joint inflammation and injury in RA8. The cleavage of the third complement protein C3 into C3a and des-ArgC3a modulate the responsiveness of MK to stromal cell-derived factor 1 (SDF-1) and regulates the increase of platelets in inflammation9.
In our previous investigations we have used a mouse model of erosive arthritis induced by intraarticular (i.a) injection of zymosan, leading to local and overall complement activation. Joint inflammation was characterized with influx of pro-inflammatory cells, giving rise to strong synovitis at the cute phase (3-5 day after zymosan injection). Then followed active phase (day 18-20) with chondrocyte proliferation, and established phase (after day 25) with chronic synovitis, cartilage and bone destruction. This model was adapted to study the complement-dependent events by the exhaustion of functional complement activity through pretreatment of mice with cobra venom factor (CVF). The results showed that the acute phase of ZIA was attenuated and the maintenance of chronic inflammation was limited 10, 11. Such approach was used in the present experiments to detect the presence of MK in inflamed joint, out of the bone marrow.
Materials and Methods
Animals
Male BALB/c mice (8-10 week old, weight 20-22 g) were housed in standard environmental conditions. All experiments were conducted in accordance with the Bulgarian Food Safety Agency Guidelines №352 06.01.2012 and approved by the Animal Care Committee at the Institute of Microbiology, Sofia.
Zymosan-Induced Arthritis (ZIA)
Mice were were divided into following groups (n=10 each in 2 separate experiments): non-arthritic, ZIA injected i.a. with 180 mg zymosan A from Saccharomyces cerevisiae (Sigma-Aldrich, Germany) and ZIA+CVF (Sigma-Aldrich, injected intraperitoneally with 10 ng/g body weight of CVF, 72 and 24 h before i.a. injection of 180 mg zymosan.
Immunohistochemistry
The paws were embedded in paraffin, serial sections were cut and mounted on glass slides. The sections were deparaffinised and after blocking of endogene peroxidase activity were incubated for 1 h with PE-conjugated anti-mouse CD62P antibody (BioLegend, San Diego, USA) and stained with with DAB solution kit (3’,3’diaminobenzididne kit, Sigma-Aldrich). The number of cells stained positive for the examined protein was determined by imaging system software (ImageJ 1.42; Research Services Branch, NIH, USA). Isotype rabbit antibody waw used as specific control.
Statistical Analysis
Data were analyzed by two way ANOVA, using InStat3.0 and GraphicPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Differences were considered significant when P<0.05.
Results
The results demonstrated that the number of CD62P positive cells increased in CVF-treated group compared to mice with ZIA at the active stage of arthritis (day 18). Giant megakaryocytes with already formed platelets were found in CVF group (Figure 1A, B and F). Such cells were absent in non-arthritic synovium (Figure 1C). The progression of arthritis resulted in significant elevation of megakaryocyte number in ZIA mice especially at the sites of synovial inflammation at the chronic phase (day 30) markedly inhibited in CVF group (Figure 1D, E and F). At day 18 the number of CD62P positive cells in cartilage was higher in CVF-treated mice compared to ZIA mice (Figure 2A, B and F) and such cells were almost absent in non-arthritic group (Figure 2C). Single positive cells were found in arthritic group at late stage of ZIA elevated in CVF group (Figure 2D, E and F).
Figure 1.Histochemically stained CD62P+ cells in the synovium. (A) arrow showed stained CD62P+ cells in ZIA at day 18; (B) arrows showed stained CD62P+ cells in ZIA+CVF at day 18 and a circle around mature MK with platelets; (C) non-arthritic group; (D) arrow showed stained CD62P+ cells in ZIA at day 30; (E) arrows showed stained CD62P+ cells in ZIA+CVF at day 30; (F) graphic presentation of results, scale bar - 20µm, *p<0.05, **p<0.01, ***p<0.001, two way ANOVA.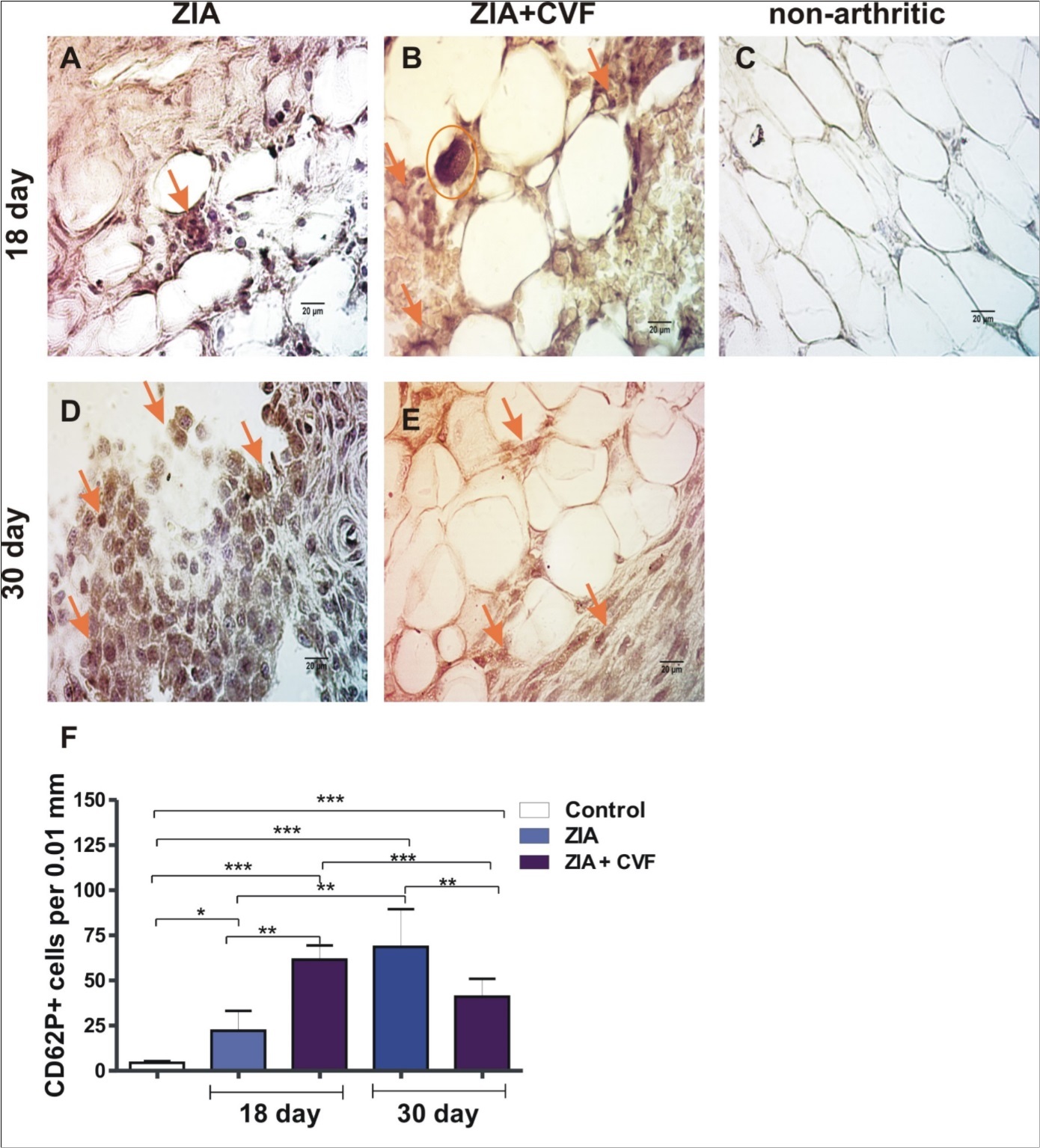
Figure 2.Histochemically stained CD62P+ cells in the cartilage. (A) arrow showed stained CD62P+ cells in ZIA at day 18; (B) arrows showed stained CD62P+ cells in ZIA+CVF at day 18; (C) non-arthritic group; (D) ) arrow showed stained CD62P+ cells in ZIA at day 30; (E) arrows showed stained CD62P+ cells in ZIA+CVF at day 30; (F) graphic presentation of results, scale bar - 20µm, *p<0.05, **p<0.01, ***p<0.001, two way ANOVA.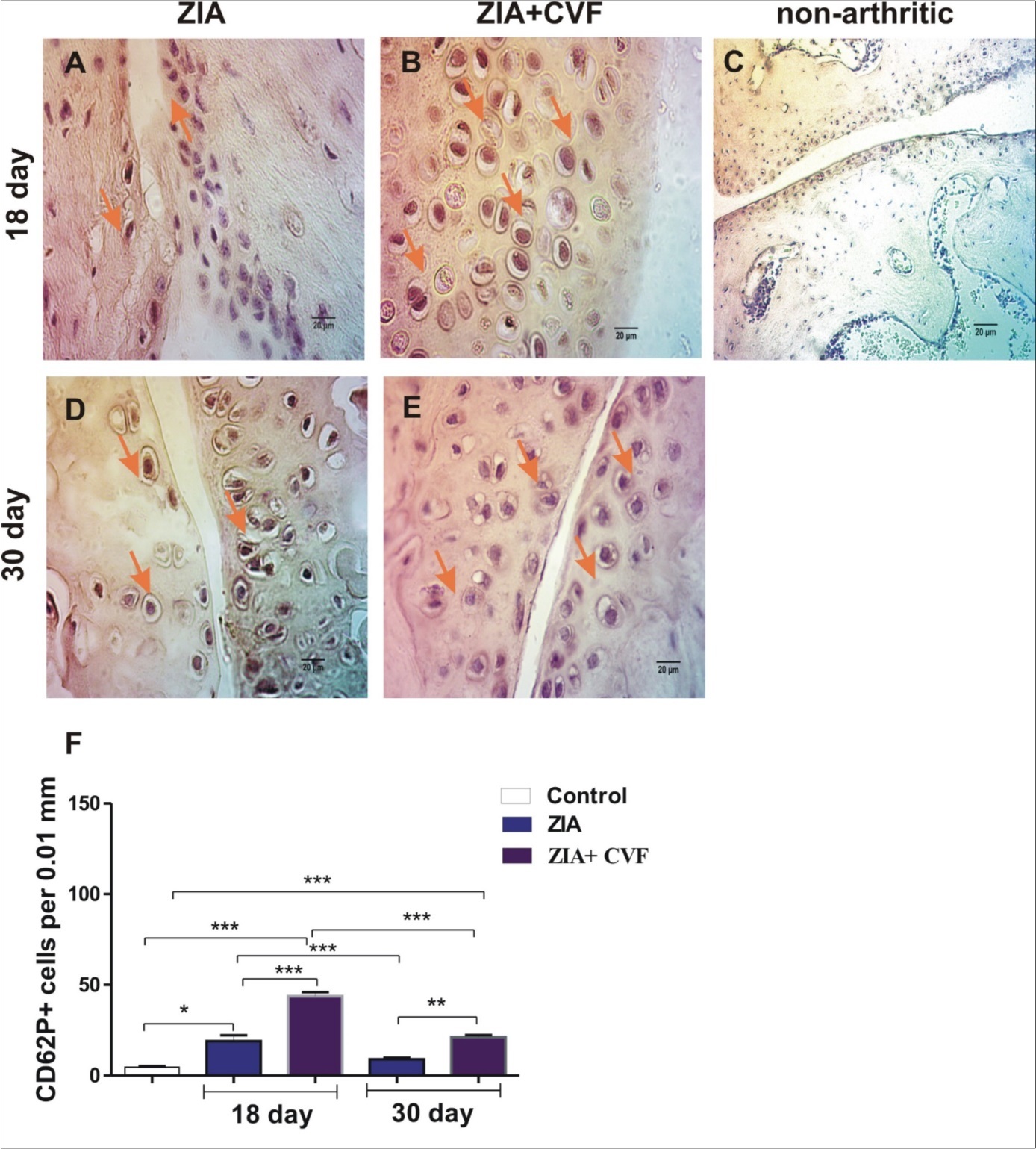
Discussion
Megakaryocytes can modulate the differentiation of osteogenic cells by the secretion of regulatory proteins, including osteocalcin, osteonectin, bone morphogenetic proteins, and OPG. The increased high local concentrations of MKs have a direct effect on osteoblastogenesis mediated by direct cell-to-cell contact of MKs and osteoblasts12. Therefore, MKs could influence bone formation and bone remodeling in a result of osteoblast proliferation and development or inhibition of OC development. There is such possibility if viable MKs persist for a longer duration in the bone marrow13. Complement activation might increase bone resorption via enhancing osteoclastogenesis, while recruitment of osteoblast precursors might favour bone formation.
Present data show that ZIA is an appropriate model to reveal the role of MKs in arthritis pathology. CD62P marker was used as it is expressed by megacaryocytes and platelets, thus we observed positive cells at different stages of MK maturation. Considering the abundance of MK in the synovium and cartilage, it is reasonable to assume that platelets by releasing cytokines and chemokines may recruit more inflammatory cells like neutrophils to the sites of inflammation. The effect of CVF-treatment differed in regard to active and established phases of ZIA. MKs, osteoblasts, and osteoclasts may interact with each other in their natural environment during joint inflmmation, as dysregulation of the MK lineage corresponds with bone pathology both in mice and in humans. Further studies are necessary to test these possibilities and to elucidate the participation of complement in MK-dependent mechanisms.
Acknowledgements.
This research was supported by a Programme supporting young scientists and PhD students of the Bulgarian Academy of Sciences, grant DFNP-17-40/26.07.2017.
References
- 1.R F Levine, K C Hazzard, J D Lamberg. (1982) The significance of megakaryocyte size.Blood. 60, 1122-1131.
- 2.Bord S, Frith E, D C Ireland, M A Scott, J I Craig. (2005) Megakaryocytes modulate osteoblast synthesis of type-l collagen, osteoprotegerin, and RANKL.Bone.36. 812-819.
- 3.Chagraoui H, Sabri S, Capron C, J L Villeval, Vainchenker W. (2003) Expression of osteoprotegerin mRNA and protein in murine megakaryocytes.ExpHematol.31. 1081-1088.
- 4.Wickenhauser C, Schmitz B, S E Baldus, Henze F, Farahmand P. (2000) Selectins (CD62L, CD62P) and megakaryocytic glycoproteins (CD41a, CD42b) mediate megakaryocyte-fibroblast interactions in human bone marrow.LeukRes.24. 1013-1021.
- 5.Aslam R, E R Speck, Kim M, A R Crow, K W Bang. (2006) Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo.Blood.107. 637-641.
- 6.Denisa D Wagner, P C Burger. (2003) . Platelets in inflammation and thrombosis.ArteriosclerThrombVascBiol.23 2131-2137.
- 7.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R. (1997) P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues.Nature.385. 81-83.
- 9.Wysoczynski M, Kucia M, Ratajczak J, M Z Ratajczak. (2007) Cleavage fragments of the third complement component (C3) enhance stromal derived factor-1 (SDF-1)-mediated platelet production during reactive postbleeding thrombocytosis.Leukemia.21. 973-982.
- 10.Belenska-Todorova L, Gyurkovska V, Ivanovska N. (2015) How complement activation influences the development of chronic synovitis in a mouse model of rheumatoid arthritis.ScandJRheumatol. 1-10.
- 11.Ganova P, Gyurkovska V, Belenska-Todorova L, Ivanovska N. (2017) Functional complement activity is decisive for the development of chronic synovitis, osteophyte formation and processes of cell senescence in zymosan-induced arthritis.Immunol. Lett.190 213-220.