Biodegradation of Methyl Parathion Using Pseudomonas stutzeri (MTCC 2643)
Abstract
Pesticides applied in agricultural fields for crop protection result in the contamination of the environment. They also affect the flora and fauna as well as the quality of air, water and soil. Hence their remediation is of concern. Among different strategies available, microbial remediation is cost effective and ecofriendly. The present work is designed to test the efficiency of Pseudomonas stutzeri, a bacterial strain obtained from MTCC, IMTECH, Chandigarh, India. After treating with 50, 100, 150 and 200 ppm of methyl parathion, parameters like, orthophosphate released, pH and turbidity changes were monitored upto thirty hours. Degradation of 200 ppm concentration was confirmed by UV-Visible spectrophotometry and HPLC analysis. This strain effectively degraded methyl parathion and it can be used in the remediation of contaminated agricultural fields.
Author Contributions
Academic Editor: Rabiul Ahasan, Professor, King Saud University, Saudi Arabia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Archana S, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Increase in population and speedy development of industrialization cause accretion of extensive assortment of chemicals in the environment. Thus, there is a need for remarkable effort to develop new technologies to reduce or remove these toxic chemical components from their accumulated area 1. Management strategies like land-filling, recycling, pyrolysis and incineration are very much effective in remediation of polluted soils. At the same time, they also cause harmful effects on the environment by leading to the development of toxic intermediates. Moreover, these methods are more luxurious and sometimes it is very difficult to implement these methods especially in large agricultural fields for example in pesticide polluted regions 2, 3, 4, 5.
Bioremediation is one of the most promising strategies which utilizes the microorganisms for the removal of pesticides from the polluted areas. It is an alternative treatment method which is cheaper, economic, flexible and environmental friendly in nature. Subsequently, it was noticed that microbes have the capacity to convert and/or degrade the xenobiotics 6, 7, 8, 9. Researchers isolated a variety of microorganisms from contaminated environment with the ability of degrading pollutants. Therefore, more research investigations have been carried out in biotransformation of organic pollutants in nature to empathize microbial environment, physiology and evolution with reference to their bioremediation ability. Microbial degradation with reference to its biochemical and genetic aspects has created more interest among the researchers which results in identification of several microorganisms with the potential to degrade pesticides along with their genes/enzymes responsible for that process. Thus, microorganisms offer prospective resources for biodegradation 10, 11, 12.
Organophosphorus pesticides including parathion, methyl parathion, malathion, monocrotophos, and dimethoate are most commonly used pesticides in agriculture all over the world. When organophosphates are dispersed in the surrounding ecosystem, their activity is determined by several environmental factors and microbial degradation. Microbial degradation is the most important factor responsible for the removal of these pesticides in the environment 13. Hence in the present study an attempt has been made to find out the methyl parathion degrading ability of Pseudomonas stutzeri strain (MTCC 2643) obtained from IMTECH, Chandigarh, Punjab, India.
Materials and Methods
Pesticide Used
The pesticide used in the present study belongs to the class of organophosphates which is commercially available as methyl parathion. It is selected on the basis of its wide application and present market trends.
Collection of Soil Sample
Pesticide, especially methyl parathion applied soil samples were collected from agricultural fields in Thiruppuvanam, Sivaganga District, Tamil Nadu, India (Figure 1) in sterile containers and immediately brought to the laboratory for analysis.
Figure 1.Location map of study area showing soil samples collection site in Tiruppuvanam, Sivaganga District, Tamil Nadu, India (Source: Google Maps)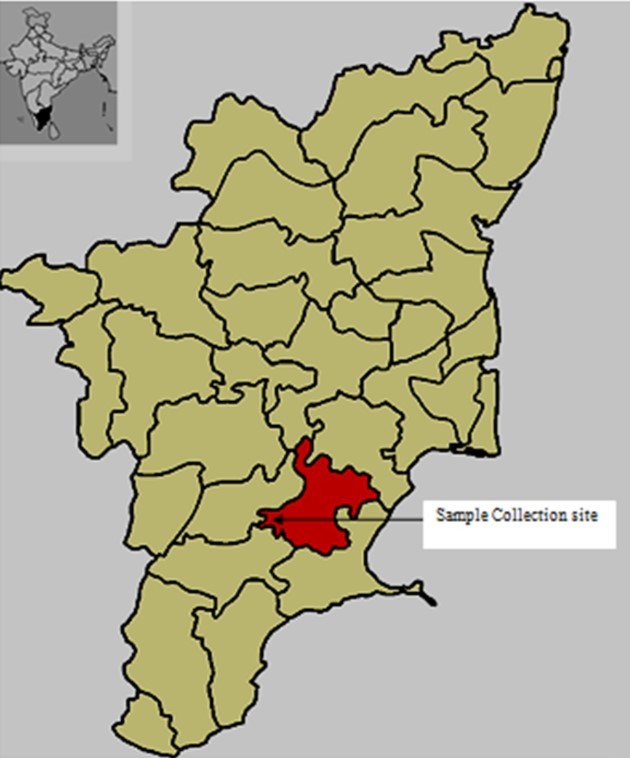
Sample Preparation
Pseudomonas stutzeri strain (MTCC 2643) obtained from IMTECH, Chandigarh, India was inoculated into minimal broth containing different concentrations of commercial grade (raw pesticide) methyl parathion (50, 100, 150 and 200 ppm). The flasks were incubated at room temperature and the samples were then subjected for the estimation of orthophosphate.
Estimation of Orthophosphate
One ml of sample was taken in a flask and 1 ml of ammonium molybdate and 3 drops of stannous chloride solution were added and kept for 10 minutes for the development of blue colour and the absorbance was recorded in a colorimeter at 650 nm. Distilled water blank was subjected in a similar manner.
Similarly the standard phosphorus solution of different strengths was processed and standard curve was plotted between absorbance and the concentrations of standard phosphorus solution. The orthophosphate content of the sample was deduced by comparing its absorbance with the standard curve.
Measurement of pH
pH was analyzed every 6 hours up to 30 hours of treatment for the samples from different concentrations of methyl parathion using pH meter and readings were recorded.
Measurement of Turbidity
Growth was measured as turbidity at 600 nm in 6 hours interval for 30 hours.
Supplementation of Sugars
The efficiency of pesticide degrading ability of the bacterial strain was tested by providing different carbon sources like fructose, glycerol, lactose, maltose and sucrose of 1% concentration in minimal medium containing 200 ppm concentration of methyl parathion. The flasks were incubated at 37°C and orthophosphate released was estimated every 6 hours up to 30 hours.
Immobilization of Cells
The seed cultures of the strain were grown in nutrient broth and the cells were harvested by centrifugation at 10,000 rpm for 10 minutes and the cells were washed and suspended in 0.1% NaCl. Then 3.5% of sodium alginate was added to the cell suspension and mixed thoroughly without forming any air bubble in the slurry. The slurry containing the cells was extended as drops through a tube (2 mm diameter) into 4% CaCl2 solution. The drops formed into spherical beads of 2 mm size. The gel beads were kept in 4% CaCl2 solution at 5°C for about an hour for complete gelation. Then the beads were washed with sterile distilled water and used for methyl parathion degradation study 14.
UV-Visible Spectrophotometry
The samples from 200 ppm concentration of methyl parathion were centrifuged at six hours interval for thirty hours and the clear supernatant was used for spectral analysis. The clear supernatant was scanned from 200 to 600 nm in a spectrophotometer (Elico SL: 159) and analysed for specific absorption in the spectrum.
High pressure Liquid Chromatographic (HPLC) Analysis
The samples from 200 ppm concentration of methyl parathion before and after 30 hours of treatment period were subjected to HPLC analysis by UV detection.
Statistical Analysis
Two way analysis of variance (ANOVA) was performed on the factors like orthophosphate released, turbidity, pH and influence of sugars for the two variables namely treatment period and methyl parathion degradation using MS-Excel package.
Results and Discussion
Pesticide contamination as a result of agricultural and industrial activities poses serious threats to the environment and indeed to human life15. In modern days, utilization of living organisms in destruction or transformation of toxic chemical compounds is very much effective when compared to traditional treatment strategies. Microbial degradation process to detoxify pesticide contaminants can be efficiently used to overcome pollution problems. Numerous reports are available indicating the use of microorganisms for pollution control and for the production of chemicals of economic importance16, 17. The pesticide methyl parathion is widely distributed in the environment due to its vast applications and is chemically similar to nerve agents used in chemical warfare18. Thus, degradation of methyl parathion is very much essential with relevant environmental concern.
Degradation of methyl parathion appears to be faster in the presence of sediment and in fresh water than that of salt water. The rate of degradation depends on the presence and acclimation of microbial populations in the body of water. Methyl parathion has a half life in water environment of 175 days and ten days to two months in soil19, 20. In the present study, the biodegradation activity of P. stutzeri MTCC 2643 was tested against methyl parathion. Orthophosphate released levels during the degradation of methyl parathion at different concentrations of methyl parathion (50, 100, 150 and 200 ppm) by P. stutzeri were analysed. P. stutzeri released maximum orthophosphate at 200 ppm concentration of methyl parathion which was selected for further analysis (Figure 2). The level of orthophosphate released in the control was very much less which indicated that P. stutzeri could utilize methyl parathion as a source of phosphorus and carbon 21. Figure 3 shows the effective degradation of methyl parathion by P. stutzeri taking place at the pH range of 6-8. With the increase in treatment period, the pH was shown to decrease at 12 hours treatment period and then decreased. From the decrease in pH, it can be understood that the acidity of the medium increases i.e, during the process of degradation, an acid was formed as a product and thus decrease in the pH was noted in the medium22. Turbidity was shown to increase during the treatment intervals such as 6, 12, 18, 24 and 30 hours (Figure 4) which indicated the growth of the organism. From the increase in the turbidity of the medium, it can be inferred that the organism uses methyl parathion as a source of carbon and phosphorus as it grows on minimal medium with methyl parathion and causing the degradation of methyl parathion. Similar kinds of observations were noticed in the earlier investigations related to biodegradation of pesticides using different bacterial strains 23, 24, 25, 26, 27.
Figure 2.Orthophosphate released during the degradation of methyl parathion by Pseudomonas stutzeri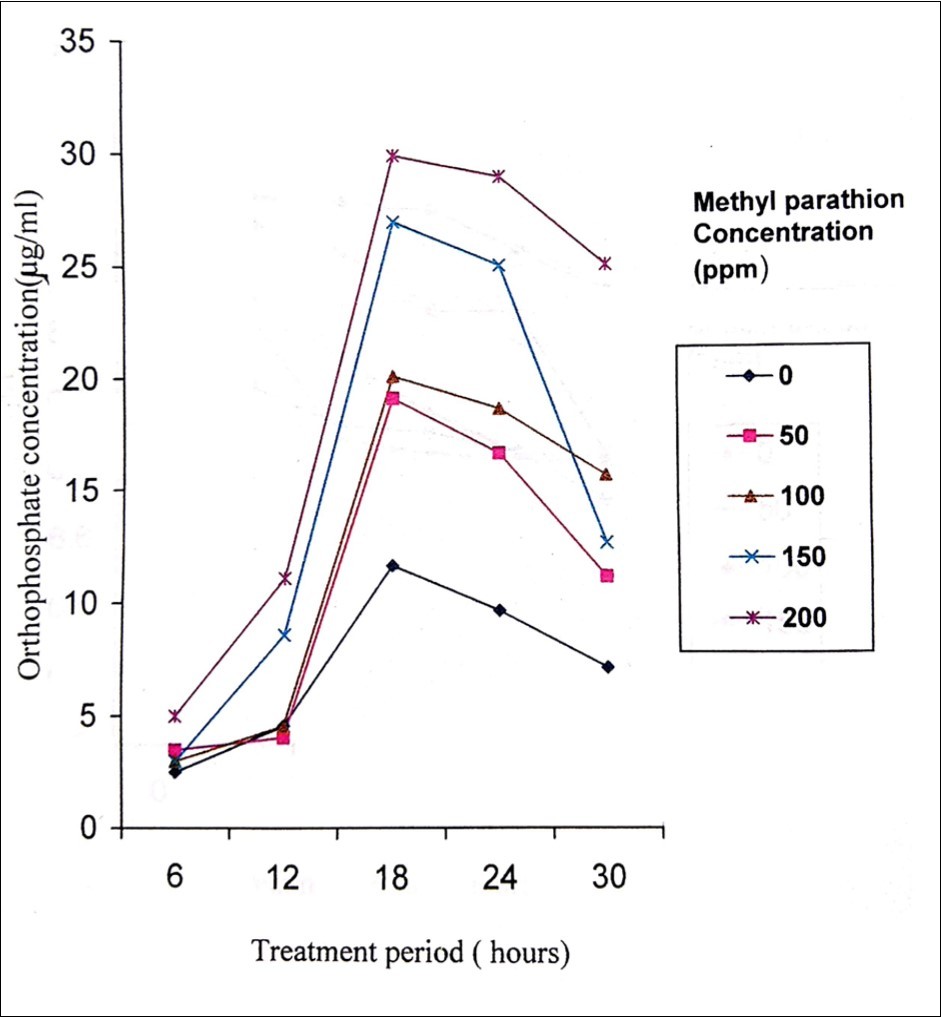
Figure 3.Changes in pH during the degradation of methyl parathion by Pseudomonas stutzeri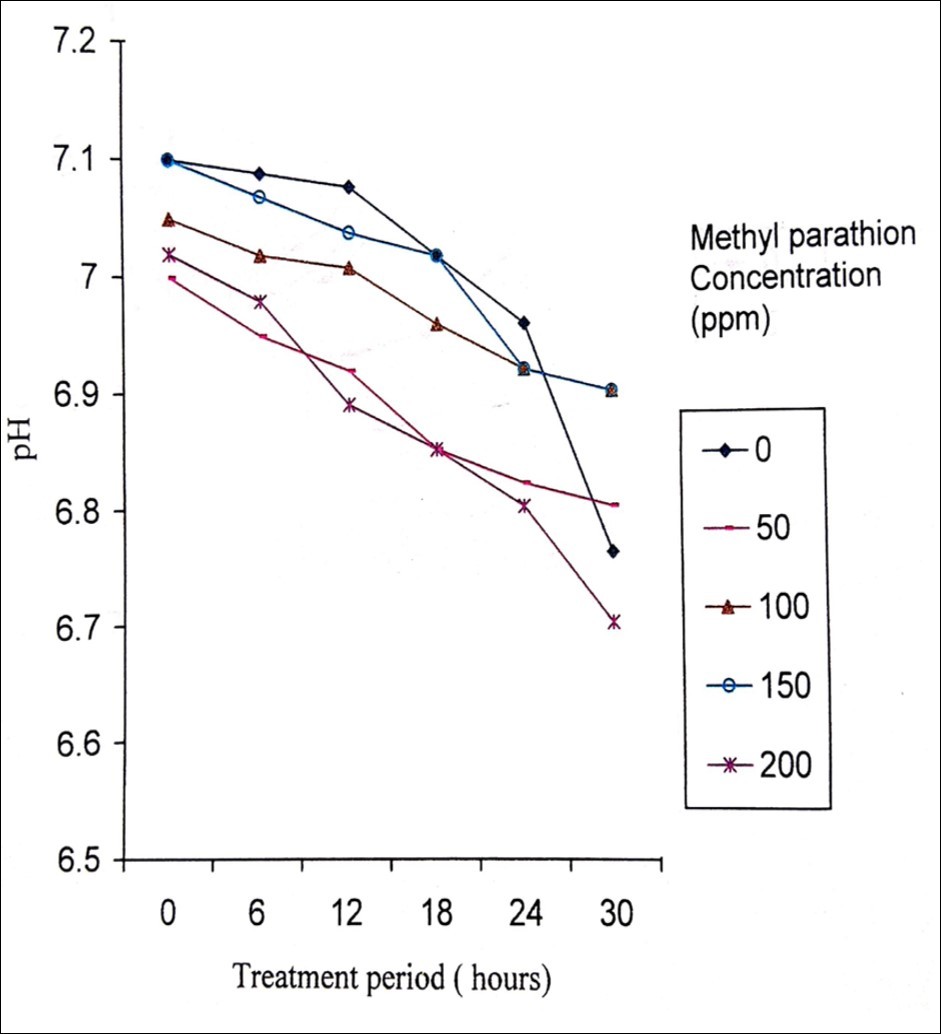
Figure 4.Turbidity during the degradation of methyl parathion by Pseudomonas stutzeri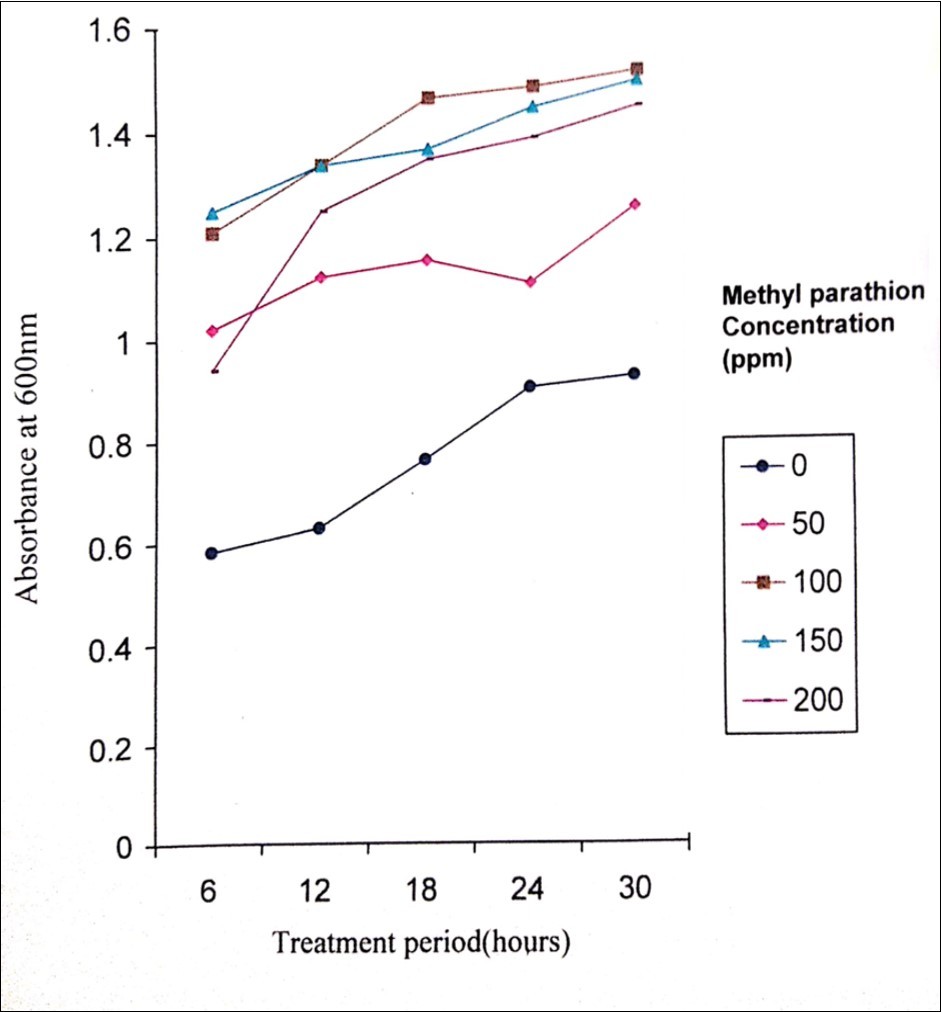
Some microorganisms can use methyl parathion as a carbon source. Methyl parathion positive effects were observed in bacteria and actinomycetes, while fungi and yeasts were less able to utilize this compound. Methyl parathion is susceptible to degradation by hydrolysis to paranitrophenol and dimethyl thiophosphate in soil and water environments by nitro group reduction to methyl amino parathion or both. Hydrolysis is a leading pathway in nonflooded soil, while methyl parathion is degraded essentially by nitro group reduction in predominantly anaerobic ecosystems such as flooded soil. Isolated two mixed bacterial cultures by soil enrichment were capable of utilizing methyl parathion as a sole source of carbon which indicated that mixed cultures are more stable in retaining their ability to completely degrade methyl parathion than that of isolated bacteria28, 29, 30, 31. Bacillus sp. was isolated from methyl parathion treated flooded soil but required yeast extract for the degradation of methyl parathion in mineral salt medium. Pseudomonas strains utilize methyl parathion as both carbon and phosphorous source. Pseudomonas sp. WBc-3 isolated from polluted soil, was capable of complete degradation of methyl parathion and tolerated high concentration of methyl parathion up to 800mg/l in basic medium and upto 2000 mg/l in 0.1% glucose medium32, 33.
In pesticide degradation, immobilized cells are more effective than that of free cells employed in conventional methods and also exhibit advantages like high cell concentrations, reuse of cells, elimination of “cell wash” problems at a high dilution rate, high yields and the capacity to retain catalytic activity for longer time 34, 35. In the present study, immobilized cells of P. stutzeri released orthophosphate with a constant, stable and gradual increase but in the case of free cells it seems to decrease after 72 hours (Figure 5). When carbon sources are supplemented in the minimal medium (fructose, glycerol, lactose, maltose, and sucrose), they enhanced the degradation process, where P. stutzeri utilized sucrose, maltose and lactose effectively for methyl parathion degradation (Figure 6).
Figure 5.Orthophosphate released during the degradation of 200ppm methyl parathion by free and immobilized cells of Pseudomonas stutzeri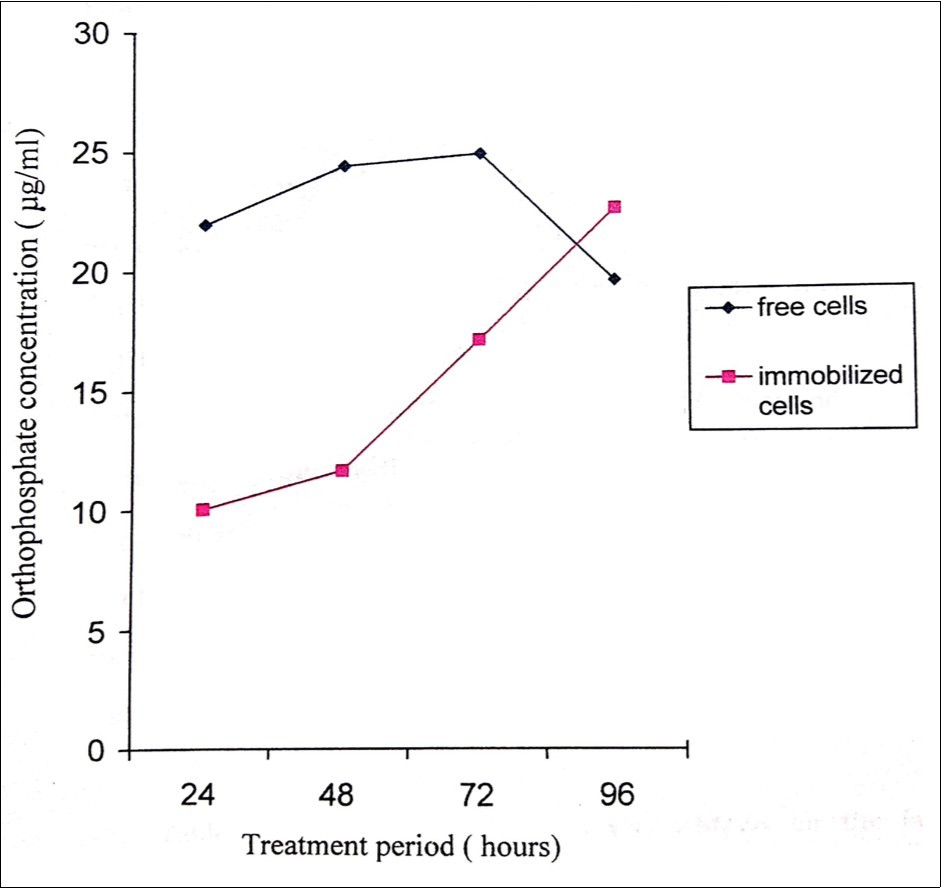
Figure 6.Orthophosphate released during the degradation of 200ppm methyl parathion by Pseudomonas stutzeri when supplemented with various sugars of 1% concentration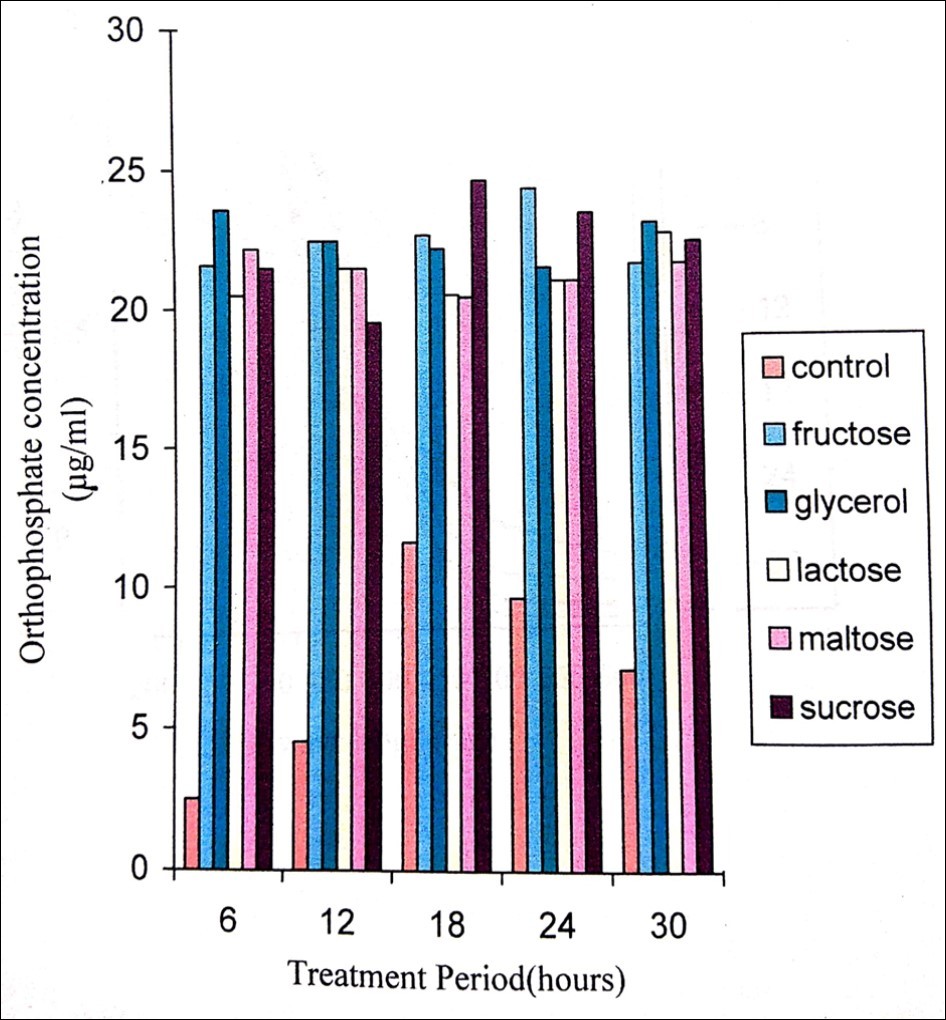
Spectrophotometry is a prominent method used in pesticide analysis over the years, because of its features like ruggedness, economical, and suitability for a variety of pesticides by using different reagents, detectors and techniques like flow injection, PLS (Partial least square) etc.36. Malik and coworkers37 have reported various methods for the analysis of fungicides spectrophotometrically. Jhangel and Pervez 38 proposed a sensitive spectrophotometric method for the analysis of the acaricide, kelthane. This method showed good sensitivity in sub parts per million levels. In the present study, UV-Visible Spectrophotometric analysis was used to find out the biodegradation of methyl parathion (Figure 7). There was a change in absorption spectrum with the increase in incubation period. This is an evidence for the degradation of methyl parathion 39, 40. Chromatographic analysis is one of the most suitable methods for pesticide analysis. HPLC is a commendable method for the analysis of wide range of pesticides. Several heat unstable and polar pesticides can be better detected with this technique. Several research works using HPLC proved its effective application in pesticide analysis 41, 42. In the present study, the retention time for standard methyl parathion was 3.433 minutes with the peak area of 7.345 mV.s and peak height of 0.444 mV (Figure 8) in HPLC analysis. The retention time for 200 ppm of methyl parathion after degradation by P. stutzeri was 2.433 minutes and 3.367 minute with the peak areas of 6.140 mV.s and 29.749 mV.s and a peak height of 0.213 mV and 1.280 mV which indicated the formation of intermediate compounds during methyl parathion degradation after 30 hours of treatment (Figure 9).
Figure 7.UV-Visible absorption spectrum taken during the degradation of 200ppm methyl parathion by Pseudomonas stutzeri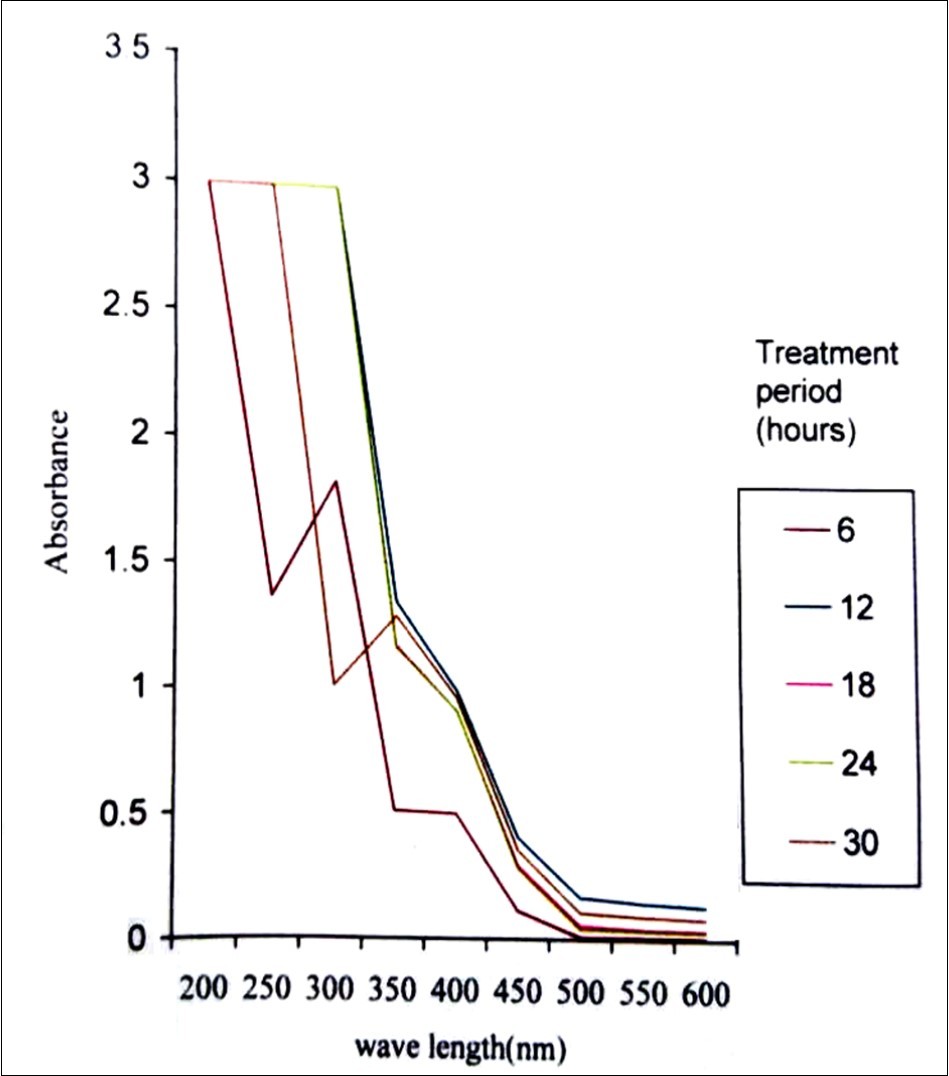
Figure 8.HPLC analysis report for 200ppm methyl parathion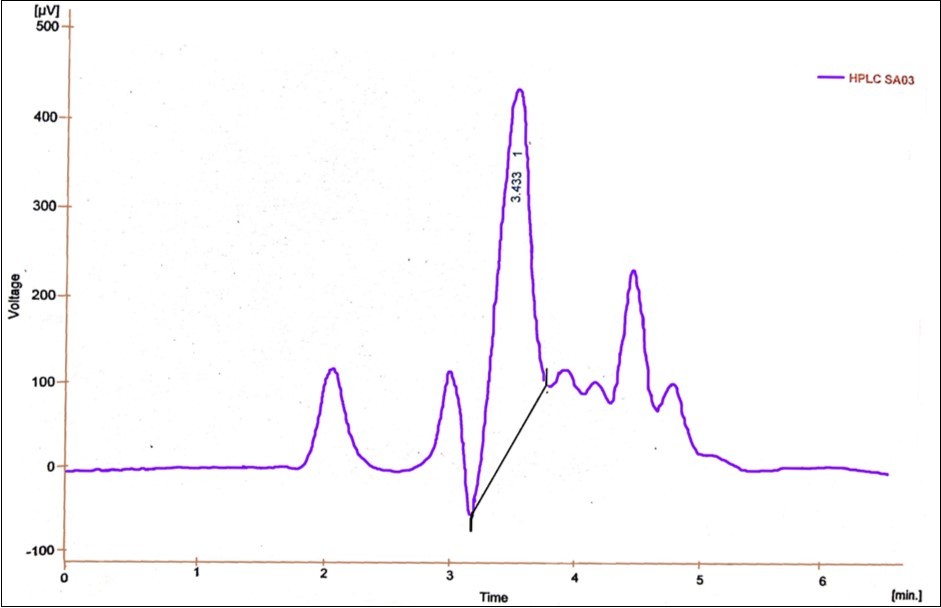
Figure 9.HPLC analysis report for 200ppm methyl parathion degradation by Pseudomonas stutzeri after 30 hours of treatment period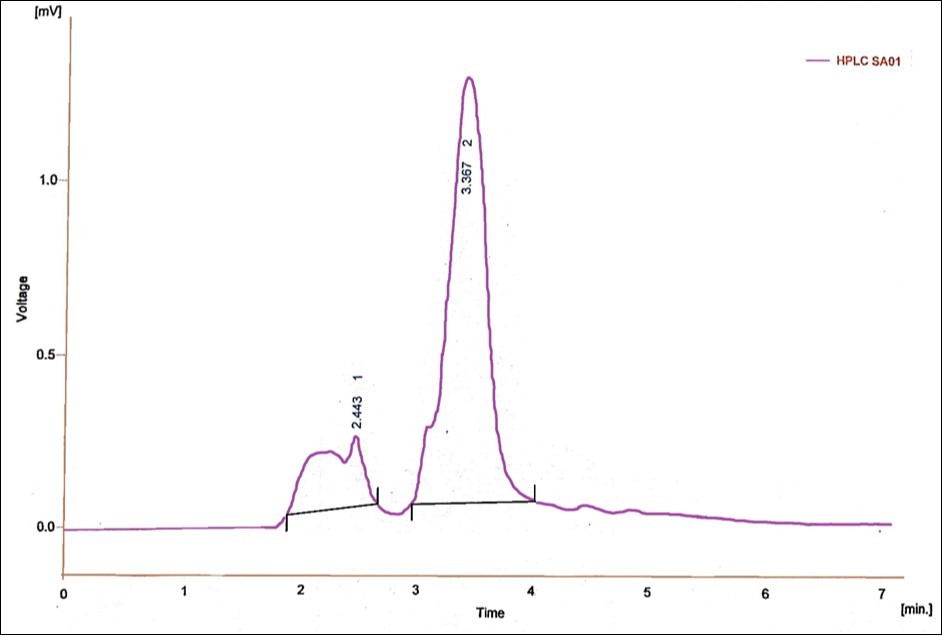
Statistical analysis of degradation rates of methyl parathion samples from two gulf coast estuaries over a three year period indicated that biodegradation occurred in the presence of sediment but was insignificant in water. Under aerobic, photosynthetic conditions, the cyanobacterium, Anabaena sp. capable of degrading methyl parathion was reported which takes place only in the presence of light43. Burkholderiacepacia which has a potential methyl parathion degrading ability from agricultural soil was isolated44. Table 1 exhibits the two way ANOVA for the factors such as orthophosphate released during degradation by P. stutzeri, pH and turbidity with the variables, treatment period and methyl parathion concentration. Variables of tested factors, treatment period and methyl parathion concentration caused significant variations which were statistically significant at 5% level. Pseudomonas stutzeri strain MTCC 2643 was able to degrade methyl parathion up to 200 ppm concentration in thirty hours. All the sugars tested enhanced the release of orthophosphate during degradation.
Table 1. Two way analysis of variance for the factors with the variables, treatment period and methyl parathion concentration| Factor | Source ofVariation | SS | df | MS | CalculatedF - value | Table valueat 5% level | Level ofsignificance |
| Orthophosphate released | Treatment Period | 475.8 | 4 | 118.9 | 9.9615 | 3.0069 | Significant P‹ 0.05 |
| Methyl Parathion Concentration | 1264.9 | 4 | 316.2 | 26.4837 | 3.0069 | Significant P‹ 0.05 | |
| pH | Treatment Period | 0.0894 | 4 | 0.022 | 47.1603 | 3.0069 | Significant P‹ 0.05 |
| Methyl Parathion Concentration | 0.0905 | 4 | 0.022 | 47.7089 | 3.0069 | Significant P‹ 0.05 | |
| Turbidity | Treatment Period | 1.4417 | 4 | 0.360 | 87.2405 | 3.0069 | Significant P‹ 0.05 |
| Methyl Parathion Concentration | 0.3199 | 4 | 0.800 | 19.3571 | 3.0069 | Significant P‹ 0.05 |
Conclusion
P.stutzeri was able to degrade methyl parathion under laboratory conditions. After strain improvement it can be used in field situations.
Acknowledgement
The authors thank the authorities of The American College, Madurai, Tamil Nadu, India, for the facilities and encouragement.
References
- 1.Suthersan S S, Horst J, Schnobrich M, Welty N, McDonough J. (2016) Remediation engineering: designconcepts.CRCPress.
- 2.X N Li, W T Jiao, R B Xiao, W P Chen, A C Chang. (2015) Soil pollution and site remediation policies in China: A review”. , Environmental Reviews 23(3), 263-274.
- 3.Pal S, A K Patra, S K Reza, Wildi W, Pote-Wembonyama J. (2010) Use of bio-resources for remediation of soil pollution”. , Natural Resources 1(2), 110-125.
- 4.R A Wuana, F E Okieimen. (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation”. IsrnEcology.
- 5.Su C. (2014) A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques”. , Environmental Skeptics and Critics 3(2), 24.
- 6.G U Chibuike, S C Obiora. (2014) Heavy metal polluted soils: effect on plants and bioremediation methods”. Applied and environmental soil science.
- 7.Megharaj M, Ramakrishnan B, Venkateswarlu K, Sethunathan N, Naidu R. (2011) Bioremediation approaches for organic pollutants: a critical perspective”. , Environment international 37(8), 1362-1375.
- 8.Kumar A, Bisht B S, Joshi V D, Dhewa T. (2011) Review on bioremediation of polluted environment: A management tool”. , International journal of environmental sciences 1(6), 1079.
- 9.K P Shukla, N K Singh, Sharma S. (2010) Bioremediation: developments, current practices and perspectives”. , GenetEngBiotechnolJ 3, 1-20.
- 10.Pant P, Pant S. (2010) A review: Advances in microbial remediation of trichloroethylene (TCE)”. , Journal of Environmental Sciences 22(1), 116-126.
- 11.Bhat M M, Shiv S, Mohammad Y, R N Shukla. (2011) Remediation of hydrocarbon contaminated soil through microbial degradation-FTIR based prediction”. Advances in Applied Science Research 2(2), 321-326.
- 12.A O Vincent, Felix E, M O Weltime, O K Ize-iyamu, Daniel E E. (2011) Microbial degradation and its kinetics on crude oil polluted soil”. , Research Journal of Chemical Science,ISSN2231-606X
- 13.Fest C, K J Schmidt. (2012) The chemistry of organophosphoruspesticides.SpringerScience&BusinessMedia.
- 14.Elakkiya M, Prabhakaran D, Thirumarimurugan M. (2016) Methods of cell immobilization and its applications”.Methods. 5(4), 211-216.
- 15.C S Jacobsen, M H Hjelmsø. (2014) Agricultural soils, pesticides and microbial diversity”. Current opinion in biotechnology. 27, 15-20.
- 16.R K Bhagobaty, S R Joshi, Malik A. (2007) Microbial degradation of organophosphorous pesticide: chlorpyrifos (mini-review)”.Internet. , Journal of Microbiology 4(1), 1-13.
- 17.Singh B, Kaur J, Singh K. (2014) Microbial degradation of an organophosphate pesticide, malathion”. Critical reviews in microbiology. 40(2), 146-154.
- 18.Kalipci E, Ozdemir C, Oztas F, Sahinkaya S. (2010) Ecotoxicological effects of methyl parathion on living things and environment”. , Afr. J. Agric. Res 5(8), 712-718.
- 19.Pino N, Peñuela G. (2011) Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site”. , International biodeterioration & biodegradation 65(6), 827-831.
- 20.Chishti Z, Hussain S, K R Arshad, Khalid A, Arshad M. (2013) Microbial degradation of chlorpyrifos in liquid media and soil”. , Journal of environmental management 114, 372-380.
- 21.R J Madhuri, Rangaswamy V. (2009) Biodegradation of selected insecticides byBacillusandPseudomonas sp. in ground nut fields”.Toxicol. , Int 16, 127-132.
- 22.Nancym P, Penuela G. (2011) Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site”.Int.Biodet.Biodegrad. 6(6), 827-831.
- 23.Qiu X, Zhong Q, Li M, Bai W, Li B. (2007) Biodegradation of p-nitrophenol by methyl parathion-degradingOchrobactrumsp. , B2”.Int,Biodet.Biodegrad 59(4), 297-301.
- 24.Debora A, A J Thatheyus, Vidhya R. (2013) Biodegradation of syntrhetic pyrethroid. , Fenvalerate byPseudomonasviridiflava”.Amc.J.Microbiol.Res 1(2), 32-38.
- 25.Debora A, A J Thatheyus, Vidhya R. (2013) Biodegradation of synthetic pyrethroid. , Fenvalerate byBacillus cereusMTCC 1305”.World J. Env.Engg 1(2), 21-26.
- 26.Ahemad M, M S Khan. (2011) Pesticide interactions with soil microflora: importance in bioremediation”. In Microbes and MicrobialTechnology.Springer , New York, NY 393-413.
- 27.M L Ortiz-Hernández, Sanchez-Salinas E, Godínez M L C, E D González, Ursino E C P. (2013) Mechanisms and strategies for pesticide biodegradation: opportunity for waste, soils and water cleaning”. , RevistaInternacionaldeContaminaciónAmbiental 29, 85-104.
- 28.Xie J, Zhao Y, Zhang H, Liu Z, Lu Z. (2014) Improving methyl parathion hydrolase to enhance its chlorpyrifos‐hydrolysing efficiency”. Letters in applied microbiology. 58(1), 53-59.
- 29.S G Parte, A D Mohekar, A S Kharat. (2017) Microbial degradation of pesticide: a review”.African journal of microbiology research. 11(24), 992-1012.
- 30.Aly M M, B A Al-aidaroos, F A Alfassi. () Pesticides Characters. Importance and Microbial Degradation”.Journal of Pharmacy and Biological Sciences.2(11): .
- 31.J K Bara, Soni R, Jaiswal S, Shrivastava K. (2017) Review on bioremediation of methyl parathion contaminated agricultural soil by microorganisms”.International. , Journal of Applied and Pure Science and Agriculture 3(5), 10-19.
- 32.Sarnaik S S. (2004) Biodegradation of organophosphorus pesticides”. Proc. Indian natn Sci Acad. B70 No 1, 57-70.
- 33.Chen Y, Zhang X, Liu H, Wang Y, Xia X. (2002) Study on Pseudomonas sp. WBC-3 capable of complete degradation of methylparathion”.Wei shengwuxuebao=. , ActamicrobiologicaSinica 42(4), 490-497.
- 34.J C Kadakol, C M Kamanavalli, Shouche Y. (2011) Biodegradation of Carbofuran phenol by free and immobilized cells ofKlebsiella pneumoniaATCC13883T”.World. , J. Microbiol.Biotechnol 271, 25-29.
- 35.M L Ortiz-Hernandez, Sanchez-Salinas E. (2010) Biodegradation of the organophosphate pesticide tetrachlorvinphos by bacteria isolated from agricultural soils in Mexico”.Rev. , Int.Contam. Ambient 26, 27-38.
- 36.Li L, Zhou S, Jin L, Zhang C, Liu W. (2010) Enantiomeric separation of organophosphorus pesticides by high-performance liquid chromatography, gas chromatography and capillary electrophoresis and their applications to environmental fate and toxicity assays”.Journal of Chromatography. 878(17), 1264-1276.
- 37.A K Malik, Kapoor J, Rao A L J. (2000) Simple and sensitive spectrophotometric determination of Ziram, Zineb and ferbam in commercial samples and food stuffs using phenylfluorone”.J.Environ.Monit. 2, 367-371.
- 38.E K Jhangal, Pervez Y. (2011) . Spectrophotometric determination of Kelthane in environmental Samples”.American J.Ana.Chem 2, 726-730.
- 39.M G Fernandez-Lopez, Popoca-Ursino C, Sanchez-Salinas E, Tinoco-Valencia R, Folch-Mallol J L et al. (2017) Enhancing methyl parathion degradation by the immobilization ofBurkholderiasp. isolated from agricultural soils”.Microbiologyopen. 6(5), 1-12.
- 40.Sharma B, Saxena S, Datta A, Arora S. (2016) Spectrophotometric Analysis of Degradation of Chlorpyrifos Pesticide by Indigenous Microorganisms Isolated from Affected Soil”.Int. , J. Current Microbiol. Applied Sci 5(9), 742-749.
- 41.Marco-Urrea E, Pérez-Trujillo M, Blánquez P, Vicent T, Caminal G. (2010) Biodegradation of the analgesic naproxen by Trametes versicolor and identification of intermediates using HPLC-DAD-MS and NMR”. , Bioresource technology 101(7), 2159-2166.
- 42.Jilani S. (2013) Comparative assessment of growth and biodegradation potential of soil isolate in the presence of pesticides”. Saudi journal of biological sciences. 20(3), 257-264.
Cited by (6)
This article has been cited by 6 scholarly works according to:
Citing Articles:
Environmental Research (2024) OpenAlex
Environmental Research (2024) Crossref
Surbhi Jaiswal, Brijeshwar Singh, Isha Dhingra, Abhijeet Joshi, Prashant Kodgire - Environmental Research (2024) Semantic Scholar
Current Genomics (2022) Crossref
Current Genomics (2022) OpenAlex
D. K. Sinha, Ayushi Gupta, A. P. Padmakumari, J. Bentur, Suresh Nair - Current Genomics (2022) Semantic Scholar