Evaluation of Anti-Aging Activity of the Biofield Energy Treated Novel Test Formulation Using SIRT1 and Telomerase Activity in in Vitro Model
Abstract
Telomerase and SIRT1 (member of the sirtuin protein family) along with the lifestyle and diet are the major determinants of aging and its associated diseases such as cancer and cardiovascular disorders. The study objective was to investigate the effect of Consciousness Energy Healing based novel test formulation in pre-adipocytes (3T3-L1) and human peripheral blood mononuclear cells (PBMCs) for anti-aging activity using SIRT1 and telomerase assay. The test formulation was divided into two parts. One portion was denoted as the untreated test item without any Biofield Energy Treatment, while the other portion was defined as the Biofield Energy Healing Treatment, which received the Biofield Energy Healing Treatment by a renowned Biofield Energy Healer, Mahendra Kumar Trivedi. The cell viability using MTT assay showed that the cell viability of 3T3-L1 and PBMCs cells was more than 70% indicating a safe and nontoxic profile. The experimental data in PBMCs cells showed that the Biofield Energy Treated Test formulation showed a significant improved telomerase activity by 39.25%, 20.86%, and 17.95% at concentrations 0.01, 5, and 100 µg/mL, respectively as compared with the untreated test formulation group. These results indicate that the Biofield Energy Healing Treatment would be the significant approach to prevent aging-related disorders such as decline cardiovascular diseases, osteoporosis, dementia, osteoarthritis, Alzheimer’s, hypertension, cancer, Parkinson's Disease, Chronic Obstructive Pulmonary Disease (COPD), Stress, Asthma, cataract, age-related macular degeneration (AMD), hearing loss and metabolic disorders.
Author Contributions
Academic Editor: Ian James Martins, Edith Cowan University, Astralia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Mahendra Kumar Trivedi, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Aging (a complex process) leads to the detrimental changes at both the molecular and cellular levels, which results in functional decline at the tissue and organ level. However, a factor such as nutrition, stress, injury, diseases and associated environmental factors affects the growth and aging 1. Physiological function has been progressively declined and is associated with dysfunction of body function leads to death. However, aging process can be slow down using scientific efforts, which can extend the lifespan and overall average lifespan. The data from WHO (World Health Organization) suggested that between 2000 and 2050 the world’s population will be doubled over 60 years of age from 11% to 22% 2. Thus, maintain the health lifestyle has been practiced by many countries, which help to prevent and postponing the aging process. Aging can be induced by various intrinsic and extrinsic factors. Alcohol intake, air pollutions, environmental pollutants, physical stress, and radiations, etc. are some extrinsic factors, while reactive oxygen species (ROS) like hydrogen peroxide (H2O2) and superoxide anions (O2−), which contain oxygen and highly reactive species, are also responsible for aging and considered as the major intrinsic factor. These factors will cause oxidative stress and damage to the biological molecules 3. Silent mating type information regulation 2 homolog (SIRTs) known as sirtuins are from NAD+ dependent enzymes family and recognized as well known modulators of lifespan in various species 4. They play an important role for the regulation of metabolic function, gene expression, apoptosis, cell survival, DNA repair, inflammation, development, and healthy aging 5, 6. Their expressions are well reported in various tissues such as liver, kidney, heart, spleen, adipose tissue, and skeletal muscle. Thus, SIRTs role has wide importance in regulation of various biological processes such as endocrine signaling, adipogenesis, and glucose and lipid metabolism and hence in aging process 7. Another important biological age factor reported to be responsible for aging is telomerase length, which is located at the extreme ends of chromosomes. Telomeric length is maintained by enzyme, telomerase. Telomerase activity is directly related with aging and correlated with age-related health diseases. However, TERT (the catalytic subunit of telomerase) expression stabilizes the telomere length and results in unlimited replicative potential of cells without any associated malignant properties 8. Accordingly, using this concept, the cellular aging can be delayed or prevented by the telomerase reactivation. However, scientific data suggested that using human cells such as blood leukocytes and PBMCs, telomerase activity can be induced 9.
Thus, a novel anti-aging test formulation was designed which consist of 7 ingredients, which is the combination of zinc chloride, ferrous sulfate, copper chloride, magnesium gluconate, pyridoxine HCl, vitamin B12 and vitamin D3. Further, the effect of test sample on SIRT1 and telomerase activity was assessed in 3T3-L1 (pre-adipocytes) and human PBMCs, respectively after Biofield Energy Healing Treatment. Biofield Energy Healing Treatment was performed because these therapies are accepted worldwide as one of the best unifying concept under Complementary and Alternative Medicine (CAM). Various Energy Healing Therapies have been reported with significant clinical and non-clinical outcomes 10, 11. Energy Healing Therapies have been practiced and accepted by the U.S. population and is well defined by National Center for Complementary and Alternative Medicine (NCCAM) 12, 13. CAM therapies such as external Johrei, therapeutic touch, Reiki, yoga, polarity therapy, Tai Chi, guided imagery, pranic healing, deep breathing, Qi Gong, chiropractic/osteopathic manipulation, meditation, massage, homeopathy, progressive relaxation, acupressure, hypnotherapy, special diets, acupuncture, relaxation techniques, healing touch, Rolfing structural integration, pilates, Ayurvedic medicine, movement therapy, mindfulness, traditional Chinese herbs and medicines in biological systems both in vitro and in vivo. The Trivedi Effect®-Consciousness Energy Healing therapies have been widely accepted worldwide in nonliving materials and living organisms. Consciousness Energy Healing Treatment found to be significant to improve the metal physicochemical properties 14, 15, 16, improved crop yield in agriculture science 17, 18, microbiology 19, 20, 21, biotechnology 22, 23, improved bioavailability of many compounds 24, 25, 26, improved skin health 27, 28, improved properties of nutraceuticals 29, 30, cancer science research 31, 32, improved overall bone health 33, 34, 35, human health and wellness. Due to the continued outcomes and wide applications of Biofield Energy Healing Treatments, the test formulation was studied for anti-aging action with respect to SIRT1 and Telomerase activity, which was assessed in 3T3-L1 (Pre-adipocytes) and human PBMCs respectively.
Materials and Methods
Chemicals and Reagents
Zinc chloride, magnesium (II) gluconate hydrate, pyridoxine hydrochloride, resveratrol, and cyanocobalamin (vitamin B12) were purchased from TCI, Japan. Iron (II) sulphate, copper chloride, and cholecalciferol (vitamin D3) were purchased from Sigma-Aldrich, USA. Telomerase assay was performed using 9 µg protein employing TeloTAGGG Telomerase PCR ELISA kit purchased from Roche Applied Science, USA. SIRT1 activity was assessed using Universal SIRT Activity Assay kit procured from Abcam, USA. Fetal bovine serum (FBS), epidermal growth factor (EGF) and Dulbecco's Modified Eagle's Medium (DMEM) were purchased from Gibco, ThermoFisher, USA. Antibiotics solution was purchased from HiMedia, India, while 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium) (MTT), direct red 80 and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma, USA. All the other chemicals used in this experiment were analytical grade procured from India.
Cell-Lines Details
For evaluation of anti-aging activity, two cell lines were used viz. mouse pre-adicocytes (3T3-L1) for SIRT1 activity and freshly isolated PBMCs for telomerase assay, respectively. 3T3-L1 cells were originated procured from embryo of Mus musculus, mouse. PBMCs cells were isolated from blood tissue from human, healthy volunteers. Both the two cell lines were maintained in the DMEM growth medium supplemented with 15% FBS, with added antibiotics penicillin (100 U/mL) and streptomycin (100 μg/mL). Standard cell line growth conditions were maintained such as 37C, 5% CO2, and 95% humidity. Positive control, resveratrol was used specifically at different non-cytotoxic concentration in MTT assay 35.
Experimental Design
The experimental test groups in anti-aging model were divided into baseline control group which included cells with DMEM, normal control group, vehicle control group (0.08% DMSO), positive control group, and the experimental tested groups at different safe concentrations. The experimental test groups included the Biofield Energy Treated and untreated test formulation with DMEM.
Energy of Consciousness Treatment Strategies
The test formulation was divided into two parts. One part each of the test formulation was treated with Biofield Energy by a renowned Biofield Energy Healer, Mahendra Kumar Trivedi remotely for ~3 minutes under standard laboratory conditions and coded as the Biofield Energy Treated test formulation. While, the second part did not receive any sort of treatment and coded as the untreated test formulation group (Control). Biofield Energy Healer in this study never visited the laboratory (Dabur Research Foundation, New Delhi, India), nor had any contact with the test formulation. This Biofield Energy Healing Treatment was provided through Healer’s unique Energy Transmission process to the test formulation. Further, the control groups were treated by a ‘sham’ healer for comparative purposes. The ‘sham’ healer did not have any knowledge about the Biofield Energy Treatment. After that, the Biofield Energy Treated and untreated samples were kept in similar sealed conditions for experimental study 35.
Estimation of Non-Cytotoxic Concentrations
The non-cytotoxic concentrations of the test samples were estimated in both the cell lines using MTT assay. 3T3-L1 cells were trypsinized, counted and then plated in 96-well plates at the density corresponding to 5 X 103 cells/well/180 µL of growth medium, while PBMCs were isolated from human blood (healthy volunteers) using HiSep(R) density gradient centrifugation method. PBMCs were counted and plated in 96-well plates at the density corresponding to 50 X 103 cells/well/180µL of growth medium. Both the cells were counted and plated in 96-well plates and were incubated overnight under specific growth conditions, which were allowed for cell recovery and exponential growth followed by serum stripping or starvation. The cells were subsequently treated with the Biofield Energy Treated and untreated test formulation at different concentrations followed by incubation from 24 to 72 hours in a CO2 incubator at 37°C, 5% CO2, and 95% humidity. Further, serum-free MTT media was added followed by incubation for 3 hours at 37°C. The supernatant were aspirated and 150 µL of DMSO was added to each well to dissolve the formazan crystals. Thereafter, at 540 nm absorbance was recorded of each well using Synergy HT micro-plate reader, BioTek, USA. The concentrations that exhibited percentage cytotoxicity of less than 30% were considered as non-cytotoxic 35.
Effect of Biofield Energy Treated Test Formulation on SIRT Activity in 3T3-L1 Cells
The 3T3-L1 cells were trypsinized, counted and then plated in 6-well plates at the density corresponding to 5 X 105 cells/well, which was then incubated overnight under standard growth conditions as to allow the cell recovery and exponential growth. The cells were then treated with positive control and Biofield Energy Treated and untreated test formulation and incubated in a CO2 incubator at 37°C, 5% CO2, and 95% humidity. After 72 hours of treatment, nuclear extracts were prepared and protein estimation was carried out for the extracts using Pierce BCA Protein Assay Kit (Thermofischer Scientific) 7. SIRT1 activity was assessed using Universal SIRT Activity Assay kit (Abcam) by manufacturer’s protocol, followed by the absorbance reading at 450 nm using Synergy HT microplate reader.
The percentage increase in SIRT1 activity was calculated using Equation 1:
% increase = (((X-T)/(R)) x 100) ----------- (1)
Where, X = SIRT1 activity corresponding to positive control and test groups after 72 hours
R = SIRT1 activity corresponding to Baseline group after 72 hours
Estimation of Telomerase Activity in PBMCs
The effect of the test formulation for the estimation of telomerase activity in PBMCs cell line was estimated. PBMCs were isolated from human blood using HiSep density gradient centrifugation method. PBMCs were counted and then plated in a 6-well plates at the density corresponding to 1.5 X 106cells/well. The above cells were then treated with positive control and Biofield Energy Treated and untreated test formulation and incubated in a CO2 incubator at 37°C, 5% CO2 and 95% humidity. After 72 hours of incubation, lysates were prepared and protein estimation was carried out for the extracts using Pierce BCA Protein Assay Kit (Thermofischer Scientific), which was used for the estimation of telomerase activity 8. Telomerase assay was performed using 9 µg protein employing TeloTAGGG Telomerase PCR ELISA kit (Roche Applied Science) as per manufacturer’s protocol followed by absorbance reading at 450 nm using Synergy HT microplate reader.
The percentage increase in the telomerase activity was calculated using Equation 2:
% increase = (((X-T)/(R)) x 100) ------------ (2)
Where, X = Absorbance of cells corresponding to positive control and test groups after 72 hours
R = Absorbance of cells corresponding to baseline group after 72 hours
Statistical Analysis
All the values for anti-aging activity were represented as percentage or specific concentrations of the respective parameters. For multiple group comparison, one-way analysis of variance (ANOVA) was used and all the statistically significant values were set at the level of p≤0.05.
Results and Discussion
Evaluation of Non-Cytotoxic Concentrations of 3T3-L1 and PBMCs Cells by MTT Assay
MTT assay was used to study the non-cytotoxic concentrations of the test formulation in both the cell lines i.e., 3T3-L1 and PBMCs. The results in term of percentage are shown in Figure 1. All the results in different groups were compared with respect to the different positive controls viz. resveratrol and phytohemagglutinin (PHA) in respective cell lines. MTT assay showed that the test formulation concentrations were found to be safe and non-toxic with more than 70% cell viability. 3T3-L1 cells showed more than 125% cell viability at 100 µg/mL test formulation concentration, which was significantly increased after Biofield Energy Treatment (Figure 1A). On the other hand, PBMCs also showed significant improved cell viability with more than 70% cell viability at 100 µg/mL test formulation concentration, which was significantly increased after Biofield Energy Treatment (Figure 1B). Thus, the non-toxic and safe test formulation concentration was further used for the assessment of anti-aging activity like SIRT assay and telomerase assay.
Figure 1.Effect of the test formulation on cell viability in 3T3-L1 and PBMCs cell lines at tested concentrations. A. 3T3-L1 cells and B. PBMCs; PHA: Phytohemagglutinin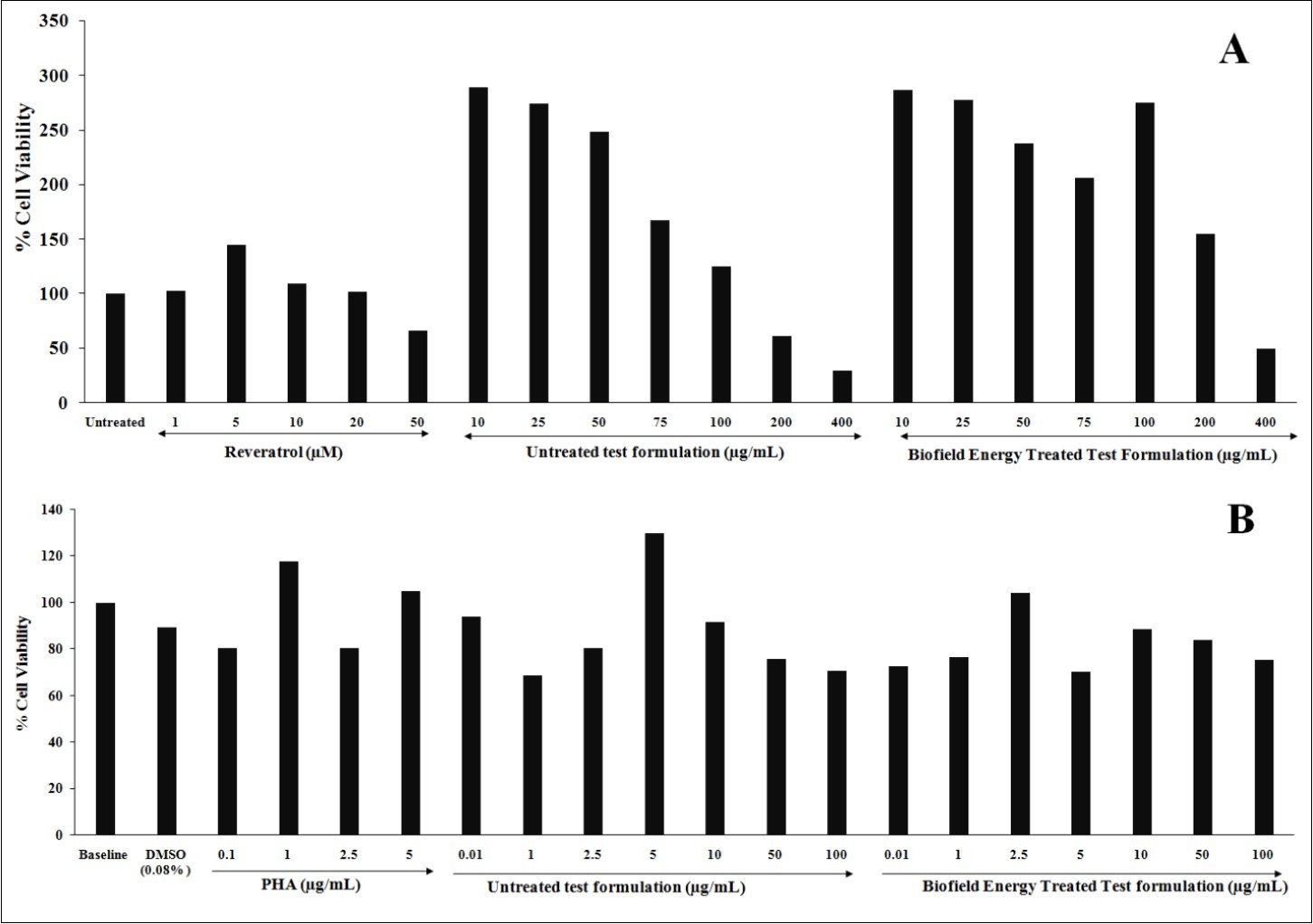
Assessment of SIRT1 Activity
The effect of test formulation and positive control was tested for SIRT1 activity and the results are illustrated in Figure 2. The positive control, resveratrol showed a significant increased SIRT1 activity by 108.56%, 112.18%, 85.67%, and 63.41% at 10, 15, 30, and 40 µM, respectively. However, the data was presented using the protein estimation in each group. The data showed that protein amount was 21.97, 23.17, 21.39, 22.12, and 18.14 µg at 10, 25, 50, 75, and 100 µg/mL, respectively in the untreated test formulation group. Similarly, Biofield Energy Treated Test formulation group data showed that protein amount was 30.04, 23.12, 21.76, 22.07, and 22.12 µg at 10, 25, 50, 75, and 100 µg/mL, respectively. However, the overall data suggested that on the basis of protein content, SIRT1 activity in terms of OD/min/mg did not show any significant increased percentage activity with respect to the untreated test formulation group.
Figure 2.Effect of the Biofield Energy Treated test formulation on SIRT1 activity in 3T3-L1 cell lines at tested concentrations.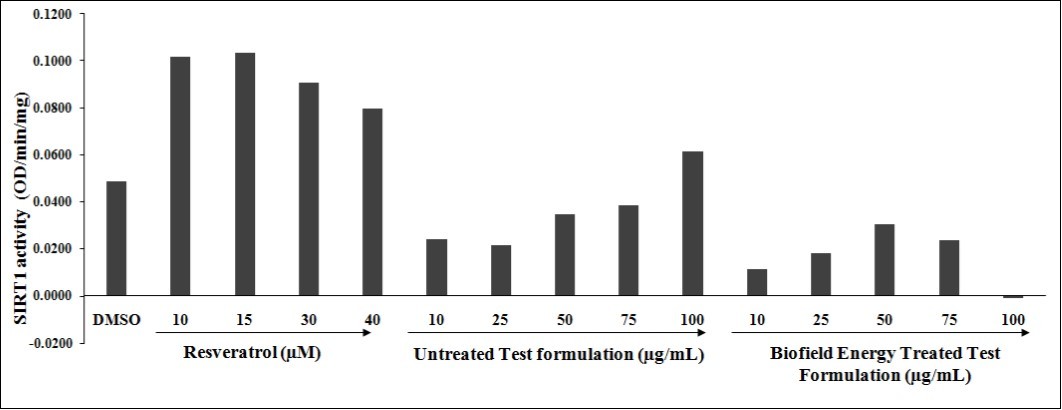
Assessment of Telomerase Activity
Telomere (enzyme which maintain telomeric length) length and aging are having direct correlation, as they are present at the extreme ends of chromosomes, which are the best indicators of biological age 36. It was reported that the increased age would result in telomere length shortening, which leads to apoptosis, cellular senescence, or oncogenic transformation of somatic cells, and significant affects the lifespan of individuals 37. Besides, it also increases the number of diseases and it was correlated with the lifestyle factors, diet, and activities that results in affecting the length of telomere. Our experimental results with Biofield Energy Treated Test formulation was studied for telomerase activity, which showed a significant improved activity after Biofield Energy Healing Treatment. The effect of test formulation and positive control was tested for telomerase activity and the results are illustrated in Figure 3. The positive control, PHA (phytohemagglutinin, µg/mL) showed a significant increased activity by 5.28%, 16.06%, and 31.75% at 0.1, 1, and 5 µg/mL, respectively as compared with the control group. However, the Biofield Energy Treated Test formulation showed a significant improved telomerase activity by 39.25%, 6.20%, 20.86%, and 17.95% at concentrations 0.01, 1, 5, and 100 µg/mL, respectively as compared with the untreated test formulation group. Thus, the overall data suggested that telomerase activity, which was directly related with aging was significantly increased that improves the overall human health. Besides, it was studied using in vitro model that the life-span can be increased by introduction of telomerase in human cell and its improved activity would reduce the age-related diseases 38.
Figure 3.Effect of the Biofield Energy Treated test formulation on telomerase activity in PBMCs cell lines at tested concentrations.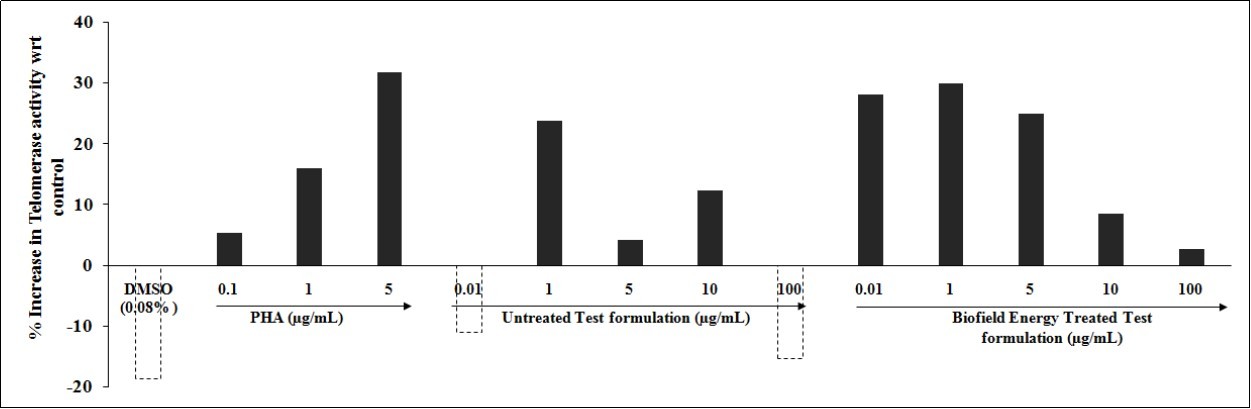
The level of telomerase activity is very important for the determination of “telomere length” in aging cells. The significance of telomerase reactivation causes cancer development and for immortalizing the cells. Thus, telomere shortening occur in the human body during aging 39. From the mechanistic point of view the telomere length is controlled by two ways: attrition and elongation. Attrition occurs as each cell divides. However, elongation process is partially modulated by the enzyme telomerase, which adds more repeating sequences to the ends of the chromosomes. In this way, telomerase could possibly reverse an aging mechanism and rejuvenates the cells 40. Thus, it can be concluded that the Trivedi Effect® would be the best approach towards anti-aging activity through improved telomerase activity by extending the telomere length.
Conclusions
The experimental results of anti-aging activity suggested that the Biofield Energy healing activity significantly improved the overall action. The activity was tested on two cells lines, and MTT data suggested that the 3T3-L1 cells has been found with more than 125% cell viability at 100 µg/mL test formulation concentration, while PBMCs showed more than 70% cell viability at 100 µg/mL test formulation concentration. Cell viability data suggested that the test formulation was found as safe and non-toxic at the tested concentrations. The telomerase activity was found to be significantly increased by 39.25%, 20.86%, and 17.95% at the concentrations of 0.01, 5, and 100 µg/mL, respectively after Biofield Energy Treated test formulation as compared with the untreated test formulation group. On the basis of experimental results the novel test formulation after treated with the Trivedi Effect®- Biofield Energy Healing significantly increased telomerase enzyme activity. Therefore, the Biofield Energy Treated test formulation can be used as a Complementary and Alternative Medicine (CAM) to prevent cardiovascular diseases, osteoporosis, dementia, osteoarthritis, Alzheimer’s type-2 diabetes, cancer, Parkinson's Disease, Chronic Obstructive Pulmonary Disease (COPD), Stress, Asthma, cataract, age-related macular degeneration (AMD), hearing loss, and metabolic disorders. In addition, various immune-mediated diseases as they all are somehow associated with age-related pathologies such as Rheumatoid arthritis, Ulcerative colitis, Lupus, Addison Disease, Celiac Disease, Graves’ Disease, Dermatomyositis, Multiple Sclerosis, Hashimoto Thyroiditis, Myasthenia Gravis, Pernicious Anemia, Aplastic Anemia, Sjogren Syndrome, Systemic Lupus Erythematosus, Diabetes, Alopecia Areata, Fibromyalgia, Vitiligo, Psoriasis, Scleroderma, Chronic Fatigue Syndrome and Vasculitis, to improve the overall health and quality of life.
Acknowledgements
The authors extend their sincere thanks and grateful to Dabur Research Foundation, India for providing the facilities and support that enabled the successful completion of the work. Authors also gratefully acknowledged to Trivedi Global, Inc., Trivedi Science, and Trivedi master wellness for their support.
Abbreviations
5-Dimethylthiazol-2-yl)-2
References
- 2.World Health Organization. (2018) Interesting facts about ageing. (accessed,August,2018).http://www.who.int/ageing/about/facts/en/.
- 3.Fusco D, Colloca G, MRL Monaco, Cesari M. (2007) Effects of antioxidant supplementation on the aging process. , Clin Interv Aging 2, 377-387.
- 4.Kaeberlein M, McVey M, Guarente L. (1999) The SIR2/3/4 complex and SIR2 alone promote longevity inSaccharomyces cerevisiaeby two different mechanisms. , Genes Dev 13, 2570-2580.
- 5.Frye R A. (1999) Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. , Biochem Biophys Res Commun 260, 273-279.
- 6.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K et al. (2007) Sirtuins: the 'magnificent seven', function, metabolism and longevity. , Ann Med 39, 335-345.
- 7.Bai L, Pang W J, Yang Y J, Yang G S. (2008) Modulation of SIRT1 by resveratrol and nicotinamide alters proliferation and differentiation of pig preadipocytes. , Mol Cell Biochem 307, 129-140.
- 8.Murillo-Ortiz B, Albarrán-Tamayo F, López-Briones S, Martínez-Garza S, Benítez-Bribiesca L et al. (2013) Increased telomere length and proliferative potential in peripheral blood mononuclear cells of adults of different ages stimulated with concanavalin A. , BMC Geriatrics 13, 99.
- 9.Iwama H, Ohyashiki K, Ohyashiki J H, Hayashi S, Yahata N et al. (1998) Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. , Hum Genet 102, 397-402.
- 10.Movaffaghi Z, Farsi M. (2009) Biofield therapies: Biophysical basis and biological regulations. , Complement Ther Clin Pract 15, 35-37.
- 11.Barnes P M, Powell-Griner E, McFann K, Nahin R L. (2004) Complementary and alternative medicine use among adults: United States. , Adv Data 343, 1-19.
- 12.Barnes P M, Bloom B, Nahin R L. (2008) Complementary and alternative medicine use among adults and children: United States. , Natl Health Stat Report 12, 1-23.
- 13.Fan K wai. (2005) National Center for Complementary and Alternative Medicine Website. , J Med Libr Assoc 93, 410-412.
- 14.Trivedi M K, Tallapragada R M. (2008) A transcendental to changing metal powder characteristics. , Met Powder 63(9), 22-28.
- 15.Trivedi M K, Nayak G, Patil S, Tallapragada R M, Latiyal O. (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. , Ind Eng Manage 4, 161.
- 16.Trivedi M K, Nayak G, Patil S, Tallapragada R M, Latiyal O et al. (2015) Effect of biofield energy treatment on physical and structural properties of calcium carbide and praseodymium oxide. , International Journal of Materials Science and Applications 4, 390-395.
- 17.Trivedi M K, Branton A, Trivedi D, Nayak G, Mondal S C et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (MangiferaindicaL.). , Journal of Food and Nutrition Sciences 3, 245-250.
- 18.Trivedi M K, Branton A, Trivedi D, Nayak G, Mondal S C et al. (2015) Evaluation of biochemical marker – Glutathione and DNA fingerprinting of biofield energy treatedOryza sativa. , American Journal of BioScience 3, 243-248.
- 19.Trivedi M K, Branton A, Trivedi D, Nayak G, Charan S et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment onCitrobacterbraakii: A urinary pathogen. , J Clin Med Genom 3, 129.
- 20.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. , J Antivir Antiretrovir 7, 083-088.
- 21.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) An impact of biofield treatment: Antimycobacterial susceptibility potential using BACTEC 460/MGIT-TB System. , Mycobact Dis 5, 189.
- 22.Trivedi M K, Patil S, Shettigar H, Bairwa K, Jana S. (2015) Phenotypic and biotypic characterization ofKlebsiellaoxytoca: An impact of biofield treatment. , J Microb Biochem Technol 7, 203-206.
- 23.Nayak G, Altekar N. (2015) Effect of biofield treatment on plant growth and adaptation. , J Environ Health Sci 1, 1-9.
- 24.Branton A, Jana S. (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in maleSprague Dawleyrats. , International Journal of Clinical and Developmental Anatomy 3, 9-15.
- 25.Branton A, Jana S. (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in maleSprague Dawleyrats. , American Journal of Clinical and Experimental Medicine 5, 138-144.
- 26.Branton A, Jana S. (2017) Effect of The biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3[25(OH)D3] in rats after a single oral dose of vitamin D3. , American Journal of Pharmacology and Phytotherapy 2, 11-18.
- 27.Kinney J P, Trivedi M K, Branton A, Trivedi D, Nayak G et al. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. , American Journal of Life Sciences 5, 65-74.
- 28.Singh J, Trivedi M K, Branton A, Trivedi D, Nayak G et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. , American Journal of Pharmacology and Phytotherapy 2, 1-10.
- 29.Trivedi M K, Branton A, Trivedi D, Nayak G, Plikerd W D et al. (2017) A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. , International Journal of Bioorganic Chemistry 2, 135-145.
- 30.Trivedi M K, Branton A, Trivedi D, Nayak G, Plikerd W D et al. (2017) Chromatographic and spectroscopic characterization of the consciousness energy healing treatedWithaniasomnifera(ashwagandha) root extract. , European Journal of Biophysics 5, 38-47.
- 31.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. , J Integr Oncol 4, 141.
- 32.Trivedi M K, Patil S, Shettigar H, Gangwar M, Jana.S (2015)In vitroevaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. , J Cancer Sci Ther 7, 253-257.
- 33.Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G et al. (2018) Influence of biofield treated vitamin D3on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. , International Journal of Biomedical Engineering and Clinical Science 4, 6-14.
- 34.Lee A C, Trivedi K, Branton A, Trivedi D, Nayak G et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). , International Journal of Nutrition and Food Sciences 7, 30-38.
- 35.Stutheit M E, Trivedi K, Branton A, Trivedi D, Nayak G et al. (2018) Biofield energy treated vitamin D3: Therapeutic implication on bone health using osteoblasts cells. , American Journal of Life Sciences 6, 13-21.
- 36.Shammas M A. (2011) Telomeres, lifestyle, cancer, and aging. , Curr Opin Clin Nutr Metab Care 14, 28-34.
- 37.Shin J S, Hong A, Solomon M J, Lee C S. (2006) The role of telomeres and telomerase in the pathology of human cancer and aging. , Pathology 38, 103-113.
Cited by (3)
This article has been cited by 3 scholarly works according to:
Citing Articles:
Current Applied Science and Technology (2022) OpenAlex
Journal of Antioxidant Activity (2021) OpenAlex
Journal of Antioxidant Activity (2021) Crossref